IntroductionCD19-directed chimeric antigen receptor (CAR) T can induce durable remissions in a fraction of relapsed/refractory large B-cell lymphomas (R/R LBCL), but 60% of patients (pts) still relapse. Biological mechanisms explaining lack of responses are emerging but they are largely unsuccessful in predicting disease response at the patient level.
AimTo address the potential role of lymphocyte features before CAR T manufacturing, we performed transcriptomic and functional evaluations of leukapheresis products (LK) in 95 R/R LBCL pts enrolled in a prospective observational study, to identify correlates of response and survival to tisagenlecleucel (Tisa-cel) and axicabtagene ciloleucel (Axi-cel).
MethodsRNA of sorted CD3+ cells from LK was digitally quantified using the nCounter CAR T Characterization Panel (NanoString). Flow cytometry (FCM) was used for CAR T enumeration and monocyte analysis. Disease response at day 90 was assessed by computed tomography (CT) and positron emission tomography-CT (PET/CT) according to Lugano criteria. Statistical analyses were performed by GraphPad Prism v.9.00 and R v.4.1.2.
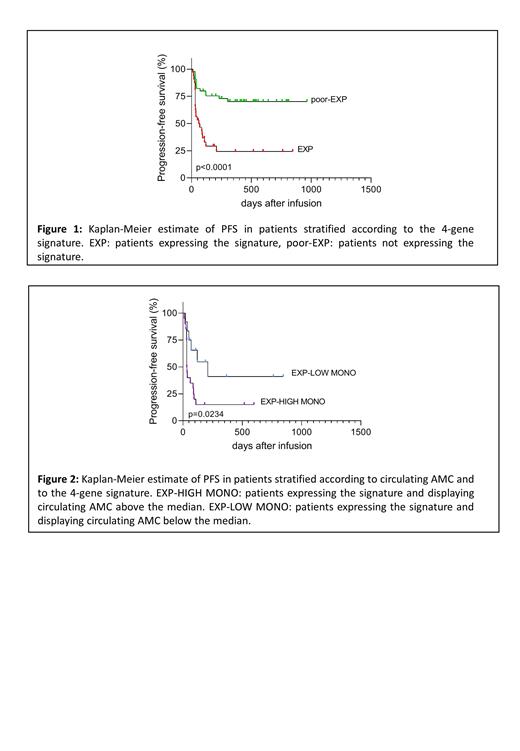
ResultsWe identified a 4-gene transcriptional signature (including MS4A4A, CD86, CD163 and SIGLEC5) able to divide LBCL pts into two groups, [namely Expressors (EXP) and poor-Expressors (poor-EXP)], characterized by significantly different survival probabilities. EXP have shorter PFS (P<0.0001: median PFS for EXP was 61 days and not reached for poor-EXP) (Fig. 1) and are less likely to respond (P<0.01). As the identified genes are highly expressed in monocytes but not in T cells, we sought to define whether monocytes contained in LK have a role in determining post CAR T outcome. In line with the fact that EXP had higher frequencies of monocytes in LK by FCM, we confirmed elevated absolute monocyte counts (AMC) both in LK (P<0.01) and in peripheral blood (PB) (P<0.05) of EXP. We then checked if monocyte levels could influence response and survival. When compared to responders, non-responders (NR) had higher LK AMC (P<0.01), higher percentages of monocytes (P<0.01) but also decreased percentages of lymphocytes (P<0.001), a parameter that was also associated with reduced PFS [HR: 0.97 (CI 0.96 - 0.99) P=0.01]. Consistent with the fact that circulating monocytes significantly correlated with those in LK (r= 0.6621, P<0.0001), NR also had significantly higher AMC (P<0.001) and AMC levels in PB negatively correlated with PFS [HR: 1.8 (CI 1.2 - 2.9) P=0.01]. Additionally, pts with serum C-reactive protein (CRP) and ferritin levels higher than the upper normal limit, displayed significantly higher AMC (P<0.001 for CRP; P<0.05 for ferritin). Accordingly, univariate analysis indicated that high ferritin, CRP and LDH levels at the time of LK were associated with the lack of response, but only high serum ferritin levels correlated with shorter PFS [HR: 2.86 (CI 1.25-6.55) P=0.013]. We next investigated if the combination of the monocyte signature and high ferritin levels and/or high circulating monocytes could better identify a group of pts at high risk of progression, compared to the expression of the 4 genes alone. Remarkably, albeit high ferritin levels and monocyte counts are overall associated with significantly reduced survival, the shortest PFS was achieved in EXP with monocyte counts above the median (P=0.0234: median PFS for EXP with high monocytes was 32.5 days and for EXP with low monocytes 209 days) (Fig. 2), thus supporting the hypothesis that CAR T treatment outcome is influenced by the presence of elevated levels of monocytes expressing the 4 genes. Concatenated monocyte FCM data from 30 pts analyzed with FlowSOM, followed by a consensus clustering algorithm combined with t-SNE (t-stochastic neighbor embedding), indicated that EXP had higher frequencies of CD14hi monocytes co-expressing MS4A4A, CD86, CD163 and SIGLEC5 at the protein level than poor-EXP (P<0.05), and accordingly, the presence of this population was also associated with lack of response (P<0.05).
ConclusionsOur data suggest that the poor outcome of some LBCL pts receiving CAR T is due to the presence of high monocytes levels expressing a four-gene signature in an inflammatory microenvironment. Additionally, the pre-manufacturing combined analysis of the gene signature and of PB AMC, could be used to plan trials evaluating CAR T cells versus other newly approved therapies such as bispecific antibodies.
Disclosures
Chiappella:Gilead-Sciences: Other: lecture fee/educational activities, advisory board; Roche: Other: lecture fee/educational activities, advisory board; Ideogen: Other: advisory board; Takeda: Other: lecture fee/educational activities, advisory board; AstraZeneca: Other: lecture fee/educational activities; Incyte: Other: lecture fee/educational activities; Jannsen-Cilag: Other: lecture fee/educational activities, advisory board; Novartis: Other: lecture fee/educational activities; Celgene-BMS: Other: lecture fee/educational activities, advisory board; SecuraBIO: Other: advisory board. Corradini:Pfizer: Other: Honoraria (Consulting, advisory role, or lecturer); Amgen: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; Celgene: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; ADC Theraputics (DSMB): Other: Honoraria (Consulting, advisory role, or lecturer); AbbVie: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; Nerviano Medical Science: Other: Honoraria (Consulting, advisory role, or lecturer); Janssen: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; Novartis: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; Kyowa Kirin: Other: Honoraria (Consulting, advisory role, or lecturer); Incyte: Other: Honoraria (Consulting, advisory role, or lecturer); Gilead/Kite: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; Daiichi Sankyo: Other: Honoraria (Consulting, advisory role, or lecturer); Roche: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; Sanofi: Other: Honoraria (Consulting, advisory role, or lecturer); SOBI: Other: Honoraria (Consulting, advisory role, or lecturer); Takeda: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; GlaxoSmithKline: Other: Honoraria (Consulting, advisory role, or lecturer); BeiGene: Honoraria; Bristol Myers Squibb: Other: Travel and accomodations.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal